INTRODUCTION TO PHYSICS.
1.MEASUREMENT OF LENGTH, MASS AND TIME.
What is Physics?
It is the branch of science which deals with the study of matter in relation to energy.
Measurement is the process of assigning numbers to observation and events.
Measurement is done with an instrument marked in a particular unit.
In mechanics, basic quantities that can be measured are length, mass and time.
There are different systems of units.
System of units known as ‘Systeme international de unites’ or SI is used in many countries in the world.
The SI unit of length is metre denoted by m.
The SI unit of mass is kilograms denoted by kg.
The SI unit of seconds is denoted by S.
MEASUREMENT OF LENGTH.
SI unit of length is metre. Other units are millimeters, centimeters, kilometers, etc.
1 metre = 100 centimeter (cm)
1 centimetre = 10 millimeters (mm)
1 kilometre = 1000 metres (m)
The metre rule.
It is used to measure length within 0.1 cm.
The vernier scale.
It has two scales; mainscale and vernier scale.
Ten scales of vernier scale covers nine scales of main scale.
Main scale division is 1∕10 cm = 0.1 cm.
Vernier scale division = 9cm ∕0.1 = 0.09 cm or 0.9 mm.
The difference between main scale and vernier scale is 0.1cm – 0.09cm = 0.01 cm.
Instruments using vernier scale includes vernier caliper, micrometer, screw gauge, Fortins barometer and travelling microscopes.
- Vernier Calipers.
It is an instrument used to measure length at the accuracy of 0.01cm. It is used to measure length in the range of 1cm to about 120cm.
It consists of a steel main scale, a fixed jaw, attached to a vernier scale which both can slide together. Lock screw is used to hold a vernier scale when readings are done.
How to read a vernier caliper.
- Ensure zero’s of main scale and vernier scales coincide.
- Open the jaws and place an object to be measured between them and hold it firmly with the jaws. Lock the vernier scale.
- Record the readings of the main scale and the vernier scale.
- Put together the readings of the main scale and vernier scale.
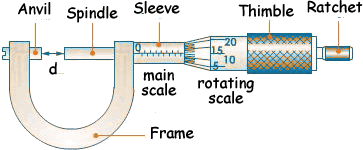
2. THE MICROMETER SCREW GAUGE.
It is used to measure length to the accuracy of 0.01cm.
It is used to measure diameters of wires and ball bearing.
It measures length up to 2.5cm.
Volume.
It is the amount of space occupied by a substance.
The SI unit of volume is cubic meters (m3)
Other units are cubic centimeters (cm3 or cc), cubic millimeters (mm3)
1m3 = 1,000,000cm3
1 litre = 1,000cm3
1 millilitre = 1cm3
Volume of liquid can be measured by using measuring cylinders, flasks, burettes and pipettes.
Mass.
Mass of a body is quantity of matter it contains.
SI unit of mass is kilogram (kg), other units used are grams and tonnes.
1 kilogram (kg) = 1000 grams.
1000 kg = 1 tonne.
Mass of a body is measured by a beam balance which compares mass of a body with known mass of another body.
Weight is measured by an instrument called spring balance.
Time.
The SI unit of time is seconds other units are minutes, hours, days, months etc.
1 minute = 60 seconds.
1 hour = 3600 seconds.
1 day = 86,400 seconds.
Weight of a body is the attractive force towards the earth’s center exerted by the earth on the body.
The magnitude of the pull of gravity on the surface of the earth is 9.8 meters per kilogram.
What is Physics?
It is the branch of science which deals with the study of matter in relation to energy.
Measurement is the process of assigning numbers to observation and events.
Measurement is done with an instrument marked in a particular unit.
In mechanics, basic quantities that can be measured are length, mass and time.
There are different systems of units.
System of units known as ‘Systeme international de unites’ or SI is used in many countries in the world.
The SI unit of length is metre denoted by m.
The SI unit of mass is kilograms denoted by kg.
The SI unit of seconds is denoted by S.
MEASUREMENT OF LENGTH.
SI unit of length is metre. Other units are millimeters, centimeters, kilometers, etc.
1 metre = 100 centimeter (cm)
1 centimetre = 10 millimeters (mm)
1 kilometre = 1000 metres (m)
The metre rule.
It is used to measure length within 0.1 cm.
The vernier scale.
It has two scales; mainscale and vernier scale.
Ten scales of vernier scale covers nine scales of main scale.
Main scale division is 1∕10 cm = 0.1 cm.
Vernier scale division = 9cm ∕0.1 = 0.09 cm or 0.9 mm.
The difference between main scale and vernier scale is 0.1cm – 0.09cm = 0.01 cm.
Instruments using vernier scale includes vernier caliper, micrometer, screw gauge, Fortins barometer and travelling microscopes.
- Vernier Calipers.
It is an instrument used to measure length at the accuracy of 0.01cm. It is used to measure length in the range of 1cm to about 120cm.
It consists of a steel main scale, a fixed jaw, attached to a vernier scale which both can slide together. Lock screw is used to hold a vernier scale when readings are done.
How to read a vernier caliper.
- Ensure zero’s of main scale and vernier scales coincide.
- Open the jaws and place an object to be measured between them and hold it firmly with the jaws. Lock the vernier scale.
- Record the readings of the main scale and the vernier scale.
- Put together the readings of the main scale and vernier scale.
2. THE MICROMETER SCREW GAUGE.
It is used to measure length to the accuracy of 0.01cm.
It is used to measure diameters of wires and ball bearing.
It measures length up to 2.5cm.

Volume.
It is the amount of space occupied by a substance.
The SI unit of volume is cubic meters (m3)
Other units are cubic centimeters (cm3 or cc), cubic millimeters (mm3)
1m3 = 1,000,000cm3
1 litre = 1,000cm3
1 millilitre = 1cm3
Volume of liquid can be measured by using measuring cylinders, flasks, burettes and pipettes.
Mass.
Mass of a body is quantity of matter it contains.
SI unit of mass is kilogram (kg), other units used are grams and tonnes.
1 kilogram (kg) = 1000 grams.
1000 kg = 1 tonne.
Mass of a body is measured by a beam balance which compares mass of a body with known mass of another body.
Weight is measured by an instrument called spring balance.
Time.
The SI unit of time is seconds other units are minutes, hours, days, months etc.
1 minute = 60 seconds.
1 hour = 3600 seconds.
1 day = 86,400 seconds.
Weight of a body is the attractive force towards the earth’s center exerted by the earth on the body.
The magnitude of the pull of gravity on the surface of the earth is 9.8 meters per kilogram.
2.Scientific process in experiment.
Physics is studied using scientific methods.
Sequence of scientific method.
1.Observation.
Involves observation of the phenomena that presents a problem, maybe an event, a situation or a fact.
2.Hypothesis formulation.
Is the guess answer to the problem asked. It is formed after collecting available information, an explanation of the phenomenon.
3.Experimentation.
Experiments are used to test hypothesis whether they are true or false.
4.Data analysis.
Helps in drawing conclusion. A person analyzing data should be capable of dealing, interpreting, and explaining any friends showing the data.
5.Drawing conclusion.
Accepting or rejecting the hypothesis depending on the result obtained from the experiment.
Physics is studied using scientific methods.
Sequence of scientific method.
1.Observation.
Involves observation of the phenomena that presents a problem, maybe an event, a situation or a fact.
2.Hypothesis formulation.
Is the guess answer to the problem asked. It is formed after collecting available information, an explanation of the phenomenon.
3.Experimentation.
Experiments are used to test hypothesis whether they are true or false.
4.Data analysis.
Helps in drawing conclusion. A person analyzing data should be capable of dealing, interpreting, and explaining any friends showing the data.
5.Drawing conclusion.
Accepting or rejecting the hypothesis depending on the result obtained from the experiment.
3. Density and Relative density.
Masses of equal volume of different materials are not equal. It is convenient to compare masses of unit volumes of materials by their densities.
Density is defined as the mass per unit volume of a substance.
Density =Mass/Volume.
SI unit of density is kilogram per cubic metre, other units are grams per cubic centimeters etc.
Example:
- A block has mass of 6kg and volume of 2m3. Find its density.
Density = mass∕volume.
Density = 6/2
Density = 3 kg/m3.
Applications of density.
- To calculate mass and weight of building materials to know the strength of supporting pillars and foundation.
- Determining purity of liquids eg. Milk.
Determination of densities of solids.
Density of rectangular solid:
Examples: Rectangular blocks, cylindrical solid, spherical solid.
Measure their mass and dimensions to calculate volume, dimensions refer to length, width, height and radius.
Volume of rectangular block
Length x width x height (lwh)
Volume of cylindrical solid
= ∏r2h
= 22/7 x r2 x h
Determination of density of liquids.
By using a measuring cylinder and beam balance.
-Measure the volume of the liquid and cylinder.
-Measure the mass of the cylinder filled with liquid.
-Measure the mass of an empty cylinder.
Density of liquid:
D = m2 – m1/ v
Data.
A clean dry beaker has mass of 200g. 200cm3 of a certain liquid are poured in a beaker with the help of a pipette. If the mass of the beaker and content is 360g, calculate the density.
Example:
Mass of beaker = 200g – M1
Liquid = 200cm3– M0
Mass and content of beaker-M2
= M2 – M1
= 360 – 200
= 160g.
D = M/V
D = 160/200
= 4/5 g/cm3
= 0.8g/cm3
Example:
A certain solid has mass of 0.5kg and volume of 0.1cm3. Calculate the density.
Density = mass/volume.
= 0.5kg/0.1m3
= 0.5 x 10/ 0.1 x 10
= 5 kg/m3
THE IMPORTANCE OF DENSITY IN LIFE.
- When selecting building materials, the density of these materials must be considered e.g. The design of ship and plane needs density factor.
- The ship is hollow and therefore it can float easily over water because the hollow space filled with air reduces its density.
- The design of equipment used in swimming needs the density factor.
Density = mass/volume.
Masses of equal volume of different materials are not equal. It is convenient to compare masses of unit volumes of materials by their densities.
Density is defined as the mass per unit volume of a substance.
Density =Mass/Volume.
SI unit of density is kilogram per cubic metre, other units are grams per cubic centimeters etc.
Example:
- A block has mass of 6kg and volume of 2m3. Find its density.
Density = mass∕volume.
Density = 6/2
Density = 3 kg/m3.
Applications of density.
- To calculate mass and weight of building materials to know the strength of supporting pillars and foundation.
- Determining purity of liquids eg. Milk.
Determination of densities of solids.
Density of rectangular solid:
Examples: Rectangular blocks, cylindrical solid, spherical solid.
Measure their mass and dimensions to calculate volume, dimensions refer to length, width, height and radius.
Volume of rectangular block
Length x width x height (lwh)
Volume of cylindrical solid
= ∏r2h
= 22/7 x r2 x h
Determination of density of liquids.
By using a measuring cylinder and beam balance.
-Measure the volume of the liquid and cylinder.
-Measure the mass of the cylinder filled with liquid.
-Measure the mass of an empty cylinder.
Density of liquid:
D = m2 – m1/ v
Data.
A clean dry beaker has mass of 200g. 200cm3 of a certain liquid are poured in a beaker with the help of a pipette. If the mass of the beaker and content is 360g, calculate the density.
Example:
Mass of beaker = 200g – M1
Liquid = 200cm3– M0
Mass and content of beaker-M2
= M2 – M1
= 360 – 200
= 160g.
D = M/V
D = 160/200
= 4/5 g/cm3
= 0.8g/cm3
Example:
A certain solid has mass of 0.5kg and volume of 0.1cm3. Calculate the density.
Density = mass/volume.
= 0.5kg/0.1m3
= 0.5 x 10/ 0.1 x 10
= 5 kg/m3
THE IMPORTANCE OF DENSITY IN LIFE.
- When selecting building materials, the density of these materials must be considered e.g. The design of ship and plane needs density factor.
- The ship is hollow and therefore it can float easily over water because the hollow space filled with air reduces its density.
- The design of equipment used in swimming needs the density factor.
Density = mass/volume.
4.FORCES.
Force is a pull or push.
When force is applied to a body at rest, it will move or tend to move the body.
- When the body is in motion, and force is applied to it, the body will change the direction of motion.
The SI unit of force is Newtons denoted by N.
TYPES OF FORCES.
1. Stretching and restoring force.
The force which results in the extension of the material is called the stretching force, the material exerts an opposing force which is called the restoring force.
2. Compressional force.
The force which results in the compression of the material or the decrease of the size of the material is known as compressional force
3. Torsional forces.
The force which results in the twisting of the material are called torsional force.
4. Attractive forces.
The forces which bring substances close to each other are attractive forces. Example: Consider bars of magnet.
5. Repulsive forces.
The forces which separate the substances which originally were close to each other are called repulsive forces.
6. Frictional forces.
The forces resulting from the rubbing of substances in contact.
Advantages:
-Walking is made possible due to frictional force.
-Braking system is possible because of frictional force.
-A moving car is stopped by the application of brakes. The parts of the machines function well due to presence of frictional force.
-To make fire using stick and matchbox, frictional force is used to produce heat.
Disadvantages:
-It can cause over heating.
-Wearing and tearing of parts of machines.
-Too much noise (unwanted noise.)
How to reduce frictional force:
-Application of lubricants e.g. Oil and grease.
-Balls and rollers.
-Smoothening the surfaces.
Note- The maximum value of frictional force exerted between two surfaces not moving relative to each other is called Limiting friction.
-The ratio of limiting friction to normal reaction is approximately constant.
Limiting friction/normal friction = constant.
The physical meaning of constant is called the coefficient of friction denoted by M.
Limiting friction/normal reaction = M.
Or F/R = M.
Note – 0 ≤ M ≤ 1.
Example:
Calculate the coefficient of friction if the friction is 20N and normal reaction is 60N.
Friction F = 20N.
Reaction R = 60N.
M= friction/reaction = F/R = 20N/60N = 1/3 = 0.33
Force is a pull or push.
When force is applied to a body at rest, it will move or tend to move the body.
- When the body is in motion, and force is applied to it, the body will change the direction of motion.
The SI unit of force is Newtons denoted by N.
TYPES OF FORCES.
1. Stretching and restoring force.
The force which results in the extension of the material is called the stretching force, the material exerts an opposing force which is called the restoring force.
2. Compressional force.
The force which results in the compression of the material or the decrease of the size of the material is known as compressional force
3. Torsional forces.
The force which results in the twisting of the material are called torsional force.
4. Attractive forces.
The forces which bring substances close to each other are attractive forces. Example: Consider bars of magnet.
5. Repulsive forces.
The forces which separate the substances which originally were close to each other are called repulsive forces.
6. Frictional forces.
The forces resulting from the rubbing of substances in contact.
Advantages:
-Walking is made possible due to frictional force.
-Braking system is possible because of frictional force.
-A moving car is stopped by the application of brakes. The parts of the machines function well due to presence of frictional force.
-To make fire using stick and matchbox, frictional force is used to produce heat.
Disadvantages:
-It can cause over heating.
-Wearing and tearing of parts of machines.
-Too much noise (unwanted noise.)
How to reduce frictional force:
-Application of lubricants e.g. Oil and grease.
-Balls and rollers.
-Smoothening the surfaces.
Note- The maximum value of frictional force exerted between two surfaces not moving relative to each other is called Limiting friction.
-The ratio of limiting friction to normal reaction is approximately constant.
Limiting friction/normal friction = constant.
The physical meaning of constant is called the coefficient of friction denoted by M.
Limiting friction/normal reaction = M.
Or F/R = M.
Note – 0 ≤ M ≤ 1.
Example:
Calculate the coefficient of friction if the friction is 20N and normal reaction is 60N.
Friction F = 20N.
Reaction R = 60N.
M= friction/reaction = F/R = 20N/60N = 1/3 = 0.33
5.ARCHIMEDES PRINCIPLE.
Experiment:
- Tie a body with a string and suspend it from a string balance.
- Find the weight of the body in air, W1.
- Find the weight of the body when partially immersed in water contained in beaker W2.
- Find the weight of the body when totally immersed in water, W3
- Remove the body from the water, dry it and find again its weight in air, W4
The results from experiment:
- W3 < W2
- W2 < W1
- W1 = W4
The weight of the body in air is greater than its weight when partially or totally immersed in water (liquid.)
Note- The loss of weight when the body is partially or totally immersed in water is NOT actual loss. This loss is called apparent loss of weight, and weight of the body when immersed in water is called apparent weight.
Apparent weight = Weight in air – Weight in liquid.
NB- Archimedes principle states that ‘’When the body is partially or totally immersed in water, the liquid exerts an upward force on the body which is equal to the weight of liquid displaced.’’
Example:
A body weighs 3N in air, when it is completely immersed in liquid, it weighs 2.2N. Find upthrust force.
Solution:
Weight in air (W1) = 3.0N
Weight in liquid (W2) = 2.2N
Apparent weight = Weight in air – Weight in liquid
=W1 – W2
=3.0N – 2.2N
=0.8N.
FLOATATION.
Note- This is a special case of Archimedes principle.
When the body floats, its apparent weight is ZERO.
W1 – W2 = 0.
Upthrust = W1 – W2 = 0.
The body is said to float on the liquid when the apparent weight is zero.
The law of floatation.
Statement- “A floating body displaces its own weight of the fluid in which it floats.”
NB- From Archimedes principle; Weight of body – apparent weight = weight of fluid displaced.
When apparent weight = 0.
Weight of body = weight of fluid displaced.
Note- When the body floats, its apparent weight = 0. Thus the weight of the floating body equals to the weight of fluid displaced and the weight of the floating body equals to the upthrust acting on it.
FLOATING SHIP.
A piece of iron sinks in water while ship made of steel floats, because a ship is very large and hollow, as a result most of its volume is filled with air which is less denser than water.
-Average density of ship is less than density of water.
The minimum height of the ship above the water level is allowed for safety of a ship. Thus minimum height depends on the type of ship and the density of water in which the ship is traveling.
T- Tropical seas
F- Fresh seas/water
S- Summer seas
W- Winter seas
TF- Tropical fresh water
WNA- Winter in North Atlantic.
Note- Ferry boats use the same principle.
BALLOONS.
It is a light bag filled with gas such as hydrogen. The density of balloon is less than the density of air. Thus the balloon will go high until it reaches a certain height where the upthrust equals to the weight of the balloon.
RELATIVE DENSITY BY ARCHIMEDES PRINCIPLE.
R.D = Mass of any volume of substance/Mass of an equal volume of water
OR
R.D = Weight of given volume of substance/Weight of an equal volume of water
OR
R.D = Weight of substance in air/Upthrust
OR
R.D = Weight of substance in air/Apparent loss of weight in water.
Example:
A body weighs 0.8N in air and 0.5N when immersed in water. Find R.D of the body.
Apparent = weight in air – weight in water
Apparent = 0.8N – 0.5N
Apparent = 0.3N
R.D = 0.8N/0.3N
= 2.7
Experiment:
- Tie a body with a string and suspend it from a string balance.
- Find the weight of the body in air, W1.
- Find the weight of the body when partially immersed in water contained in beaker W2.
- Find the weight of the body when totally immersed in water, W3
- Remove the body from the water, dry it and find again its weight in air, W4
The results from experiment:
- W3 < W2
- W2 < W1
- W1 = W4
The weight of the body in air is greater than its weight when partially or totally immersed in water (liquid.)
Note- The loss of weight when the body is partially or totally immersed in water is NOT actual loss. This loss is called apparent loss of weight, and weight of the body when immersed in water is called apparent weight.
Apparent weight = Weight in air – Weight in liquid.
NB- Archimedes principle states that ‘’When the body is partially or totally immersed in water, the liquid exerts an upward force on the body which is equal to the weight of liquid displaced.’’
Example:
A body weighs 3N in air, when it is completely immersed in liquid, it weighs 2.2N. Find upthrust force.
Solution:
Weight in air (W1) = 3.0N
Weight in liquid (W2) = 2.2N
Apparent weight = Weight in air – Weight in liquid
=W1 – W2
=3.0N – 2.2N
=0.8N.
FLOATATION.
Note- This is a special case of Archimedes principle.
When the body floats, its apparent weight is ZERO.
W1 – W2 = 0.
Upthrust = W1 – W2 = 0.
The body is said to float on the liquid when the apparent weight is zero.
The law of floatation.
Statement- “A floating body displaces its own weight of the fluid in which it floats.”
NB- From Archimedes principle; Weight of body – apparent weight = weight of fluid displaced.
When apparent weight = 0.
Weight of body = weight of fluid displaced.
Note- When the body floats, its apparent weight = 0. Thus the weight of the floating body equals to the weight of fluid displaced and the weight of the floating body equals to the upthrust acting on it.
FLOATING SHIP.
A piece of iron sinks in water while ship made of steel floats, because a ship is very large and hollow, as a result most of its volume is filled with air which is less denser than water.
-Average density of ship is less than density of water.
The minimum height of the ship above the water level is allowed for safety of a ship. Thus minimum height depends on the type of ship and the density of water in which the ship is traveling.
T- Tropical seas
F- Fresh seas/water
S- Summer seas
W- Winter seas
TF- Tropical fresh water
WNA- Winter in North Atlantic.
Note- Ferry boats use the same principle.
BALLOONS.
It is a light bag filled with gas such as hydrogen. The density of balloon is less than the density of air. Thus the balloon will go high until it reaches a certain height where the upthrust equals to the weight of the balloon.
RELATIVE DENSITY BY ARCHIMEDES PRINCIPLE.
R.D = Mass of any volume of substance/Mass of an equal volume of water
OR
R.D = Weight of given volume of substance/Weight of an equal volume of water
OR
R.D = Weight of substance in air/Upthrust
OR
R.D = Weight of substance in air/Apparent loss of weight in water.
Example:
A body weighs 0.8N in air and 0.5N when immersed in water. Find R.D of the body.
Apparent = weight in air – weight in water
Apparent = 0.8N – 0.5N
Apparent = 0.3N
R.D = 0.8N/0.3N
= 2.7
6.PROPERTIES OF MATTER.
Matter is anything that has mass and occupies space. Examples: stone, water, house, paper.
STATES OF MATTER.
There are three main states of matter, namely:
Solid
Liquid
Gas.
Solids:
Substances like sand, stone, wood, iron etc.
Liquids:
Substances like water, kerosene, mercury etc.
Gas:
Substances like oxygen, carbondioxide, etc.
Note- Solids have definite shape and size.
Liquids have definite volume but take the shape of the container.
Gases have neither definite size nor shape, their volumes are equal to the volume of the container.
STRUCTURE OF MATTER.
Any matter is made up of tiny particles like atoms or molecules.
SOLIDS.
- The molecules are held together by strong attractive forces
- Molecules are not free to move but can vibrate in a fixed position.
- The molecules are closely packed together.

LIQUIDS.
- In liquids there are weak forces of attraction between the molecules.
- Molecules are free to move randomly.
- Intermolecular spaces are greater between molecules.
GASES.
- Molecules have very weak forces of attraction.
- Molecules are very free to move.
- The intermolecular spaces are greater than those in solids and liquids.
BROWNIAN MOVEMENT.
An English botanist, Robert Brown poured some pollen grains in water, the particles floating in water were darting about. The irregular motion of the tiny particles suspended in fluid is called Brownian movement. This irregular motion is due to the fact that the tiny particles are bombarded by the liquid or gas molecules which are in a state of motion.
ELASTICITY.
When the substance is stretched, the dimensions change. When the stretching force is removed, the substance returns to its original shape and size.
The property of the substance to recover its original shape and size after the removal of the stretching force is called ELASTICITY.
SURFACE TENSION.
The property of the liquid to form a skin like structure at the surface is surface tension.
OBSERVATION:
Mosquito larvae in water support themselves from the surface of water.
A pond surface is capable of walking on the surface of water due to the presence of surface tension. A steel needle can float on the water due to the presence of surface tension.
FACTORS AFFECTING SURFACE TENSION.
- When the impurities are added to the liquid, the surface tension decreases.
- When the temperature is increased, the surface tension lowers.
- Soap reduces the surface tension of the liquid.
- Oil/kerosene and other detergents reduce surface tension.
COHESIVE AND ADHESIVE FORCES.
Molecules of any substances are held together by attractive forces.
COHESIVE FORCES.
These are attractive forces between the molecules of the same substance e.g. Water molecules or glass molecules.
ADHESIVE FORCES.
These are attractive forces between the molecules of different substances e.g. Water and glass molecules
REMEMBER:
- A drop of water spreads over the surface of the glass because the forces of adhesion are greater than those of cohesion.
- The drop of mercury remains spherical because the forces of cohesion are greater than forces of adhesion. That’s why the mercury does not wet the glass.
CAPILLARITY.
It is the use or fall of liquids in narrow tubes.
A capillary tube is a glass tube with a very fine bore. Water uses inside the tube when the tube is dipped in water. When dipped in mercury, there is a depression of mercury due to the fact that the forces of cohesion between the molecules of mercury overcome those of adhesion between mercury and glass.
Note:
- The absorption of water by towel.
- The using up of kerosene in the wick of a lamp.
- The rising of water in soil.
- Blotting paper on ink.
Remember: The meniscus of water opens upwards and meniscus of mercury opens downwards.
VISCOSITY.
It is the friction which occurs in fluids or the existence of force of friction between the layers of a liquid or a gas.
Note- Different liquids have different properties. It is more difficult to stir oil than to stir water. This is due to viscosity. The liquids which are difficult to stir and don’t flow easily are called viscous liquids. When a spherical metal ball is placed on the surface of the liquid, it sinks down with a viscosity which increases as it sinks.
Note- Terminal velocity is the maximum velocity reached by a falling body when the sum of upward forces is equal to the weight of the body. A parachute falling freely under gravity attains a terminal velocity after it has fallen for sometime.
DIFFUSION.
Is the process by which different gases or liquids mix.
Note – Oxygen in lungs diffuses into the blood stream during respiration.
 – When a bottle of perfume is opened, the smell spreads over a vast area mixing with air by diffusion.
– When a bottle of perfume is opened, the smell spreads over a vast area mixing with air by diffusion.
OSMOSIS.
The passage of water from high water concentration to low water concentration through a semi permeable membrane.
Demonstration:
A deep scoop is made into an Irish potato, half filled with sugar solution and placed in a vessel containing water.
A scoop is observed to contain water from the vessel. Tie a cellophane membrane to a thistle funnel, fill the funnel with concentrated sugar solution and then immerse it in water.
Observation:
It is observed that the level of the liquid in the stem of the funnel begins to rise immediately. The maximum increase in height of the solution in the thistle is a measure of the Osmotic Pressure.
PRESSURE.
It is the force exerting normally per unit area.
Mathematically:
Pressure = Force/area
Symbolically:
P= F/A
SI unit of pressure is as follows:
Force is expressed in Newtons.
Area is expressed in metre square (m2)
P = Force (N)/Area (m2)
Thus SI unit of pressure is Newton per metre squared. N/m2
Sometimes expressed as Pascal, Pa.
Matter is anything that has mass and occupies space. Examples: stone, water, house, paper.
STATES OF MATTER.
There are three main states of matter, namely:
Solid
Liquid
Gas.
Solids:
Substances like sand, stone, wood, iron etc.
Liquids:
Substances like water, kerosene, mercury etc.
Gas:
Substances like oxygen, carbondioxide, etc.
Note- Solids have definite shape and size.
Liquids have definite volume but take the shape of the container.
Gases have neither definite size nor shape, their volumes are equal to the volume of the container.
STRUCTURE OF MATTER.
Any matter is made up of tiny particles like atoms or molecules.
SOLIDS.
- The molecules are held together by strong attractive forces
- Molecules are not free to move but can vibrate in a fixed position.
- The molecules are closely packed together.

LIQUIDS.
- In liquids there are weak forces of attraction between the molecules.
- Molecules are free to move randomly.
- Intermolecular spaces are greater between molecules.
GASES.
- Molecules have very weak forces of attraction.
- Molecules are very free to move.
- The intermolecular spaces are greater than those in solids and liquids.
BROWNIAN MOVEMENT.
An English botanist, Robert Brown poured some pollen grains in water, the particles floating in water were darting about. The irregular motion of the tiny particles suspended in fluid is called Brownian movement. This irregular motion is due to the fact that the tiny particles are bombarded by the liquid or gas molecules which are in a state of motion.
ELASTICITY.
When the substance is stretched, the dimensions change. When the stretching force is removed, the substance returns to its original shape and size.
The property of the substance to recover its original shape and size after the removal of the stretching force is called ELASTICITY.
SURFACE TENSION.
The property of the liquid to form a skin like structure at the surface is surface tension.
OBSERVATION:
Mosquito larvae in water support themselves from the surface of water.
A pond surface is capable of walking on the surface of water due to the presence of surface tension. A steel needle can float on the water due to the presence of surface tension.
FACTORS AFFECTING SURFACE TENSION.
- When the impurities are added to the liquid, the surface tension decreases.
- When the temperature is increased, the surface tension lowers.
- Soap reduces the surface tension of the liquid.
- Oil/kerosene and other detergents reduce surface tension.
COHESIVE AND ADHESIVE FORCES.
Molecules of any substances are held together by attractive forces.
COHESIVE FORCES.
These are attractive forces between the molecules of the same substance e.g. Water molecules or glass molecules.
ADHESIVE FORCES.
These are attractive forces between the molecules of different substances e.g. Water and glass molecules
REMEMBER:
- A drop of water spreads over the surface of the glass because the forces of adhesion are greater than those of cohesion.
- The drop of mercury remains spherical because the forces of cohesion are greater than forces of adhesion. That’s why the mercury does not wet the glass.
CAPILLARITY.
It is the use or fall of liquids in narrow tubes.
A capillary tube is a glass tube with a very fine bore. Water uses inside the tube when the tube is dipped in water. When dipped in mercury, there is a depression of mercury due to the fact that the forces of cohesion between the molecules of mercury overcome those of adhesion between mercury and glass.
Note:
- The absorption of water by towel.
- The using up of kerosene in the wick of a lamp.
- The rising of water in soil.
- Blotting paper on ink.
Remember: The meniscus of water opens upwards and meniscus of mercury opens downwards.
VISCOSITY.
It is the friction which occurs in fluids or the existence of force of friction between the layers of a liquid or a gas.
Note- Different liquids have different properties. It is more difficult to stir oil than to stir water. This is due to viscosity. The liquids which are difficult to stir and don’t flow easily are called viscous liquids. When a spherical metal ball is placed on the surface of the liquid, it sinks down with a viscosity which increases as it sinks.
Note- Terminal velocity is the maximum velocity reached by a falling body when the sum of upward forces is equal to the weight of the body. A parachute falling freely under gravity attains a terminal velocity after it has fallen for sometime.
DIFFUSION.
Is the process by which different gases or liquids mix.
Note – Oxygen in lungs diffuses into the blood stream during respiration.

– When a bottle of perfume is opened, the smell spreads over a vast area mixing with air by diffusion.
OSMOSIS.
The passage of water from high water concentration to low water concentration through a semi permeable membrane.
Demonstration:
A deep scoop is made into an Irish potato, half filled with sugar solution and placed in a vessel containing water.
A scoop is observed to contain water from the vessel. Tie a cellophane membrane to a thistle funnel, fill the funnel with concentrated sugar solution and then immerse it in water.
Observation:
It is observed that the level of the liquid in the stem of the funnel begins to rise immediately. The maximum increase in height of the solution in the thistle is a measure of the Osmotic Pressure.
PRESSURE.
It is the force exerting normally per unit area.
Mathematically:
Pressure = Force/area
Symbolically:
P= F/A
SI unit of pressure is as follows:
Force is expressed in Newtons.
Area is expressed in metre square (m2)
P = Force (N)/Area (m2)
Thus SI unit of pressure is Newton per metre squared. N/m2
Sometimes expressed as Pascal, Pa.
7.PRESSURE IN LIQUIDS.
- VARIATION OF LIQUID PRESSURE WITH DEPTH.
The more the depth, the higher the pressure at any point.
- VARIATION OF LIQUID PRESSURE WITH DENSITY.
The greater the density of the liquid, the greater the pressure and vice versa.
- PRESSURE AT ANY POINT OF THE LIQUID.
The pressure at any point in a liquid acts equally in all directions.
- A LIQUID FINDS ITS OWN LEVEL.
For any liquid the pressure at any point within its varies only with the vertical depth of the point below the surface of the liquid.
The liquid stands at the same vertical height in all the tubes whatever their shapes.
CALCULATION OF PRESSURE IN LIQUIDS.
Mass x acc.due to gravity = weight.
Mg= w
Weight = S gh
P = F/A = SAhg/A
P = S gh
Where S = Density of liquids.
g = acc.due to gravity
h = height/depth.
Example:
Given that the density of the liquid is 1000g/m contained to a height of 4m, calculate the pressure exerted by the liquid column is acceleration due to gravity, g = 10m/s2
Data:
S= 1000k/m2
H= 4m
G= 10m/s2
P = Sgh
= 1000 x 10 x 4
= 40,000 n/m2
MEASUREMENTS.
Measure heights h1 and h2 with reference to the surface of separation at B such that B and C are on the same horizontal level of the same liquid, this is true that the pressures at B and C are equal, since the atmospheric pressure is same on each side of the U-tube.
CONCLUSION:
The pressure at B due to liquid column AB equals to the pressure at C due to liquid column CD.
Let S1 be density of water and S2 be density of paraffin.
S1 h1g = S2 H2g
S1 H1 = S2 H2
S1 = S2 H2/ H2
SI/S2 = H2/H1
Note: If the density of water is 1000kg/m2, h1 and h2 are measured, then s2 can be calculated. An inverted U-tube known as Hare’s apparatus is used to compare the densities of two liquids which mix together.
Dip the two open tubes of Hare’s apparatus into beakers containing water and the liquid of the unknown density when the clip is opened to suck out the air, the liquids use to different heights and then remain there when the clip is closed.
– Measure h1 and h2.
Then P = S1h2g
S1h1g = S2h2g
S1h1 = S2h2
S1/h2 = h2/h1
Unknown density S2 can be calculated.
TRANSMISSION OF PRESSURE IN FLUIDS.
Liquids and gases are fluids because they can flow. Pascal’s principle states that ‘’When pressure is applied at any point on the surface of a fluid contained in closed vessel, the pressure is transmitted undiminished to all parts of the fluid and to the walls of the vessel.”
Uses of principle:
- Used in mechanical diggers
- Used in bull-dozers
- Used in hydraulic press
- Used in jacks
THE HYDRAULIC PRESS.
Pressure at small piston=F1/A1
Pressure at large piston=F2/A2
A force of 5N is applied to smaller piston. If the smaller piston has cross sectioned area of 0.001m2 and the larger piston has cross sectioned area of 0.1m2. Find the force of the larger piston.
f/a = F/A
5N/0.001 = F/0.1
5 X 0.1 = 0.001 F
F = 0.5/0.001 = 500N
F = 500N.
THE CAR BRAKES.
In modern cars, the braking systems retard the wheels equally to minimize the dangers of skidding or locking the wheels when the brakes are applied.
The driver presses the car using a small force to the master piston P which moves forward, the pressure exerted at the master piston P is transmitted, undiminished through the oil pipes to the piston of brake cylinder C, the piston N in the brake cylinder moves forward against the return spring thus bringing brake shoes B into contact with the brake drum D. This stops the wheels of the car.
MANOMETER.
A device used to measure gas pressure.
It consists of a U-tube containing a liquid, when both arms are open to the atmosphere, the same atmospheric pressure is exerted on the liquid surface A and B and there will be at the same horizontal level.
Side A is connected to the gas supply when the top is turned on, the gas exerts pressure on the surface A with the level B rises and A falls until the pressure at C in side the liquid becomes equal to the gas pressure i.e Shg, h is liquid heat.
Side A is connected to the gas supply, when the top is turned on the gas exerts pressure on surface A with the result that level B rises and A falls until the pressure at C inside the liquid becomes equal to the gas pressure i.e Shg, his liquid heat.
- VARIATION OF LIQUID PRESSURE WITH DEPTH.
The more the depth, the higher the pressure at any point.
- VARIATION OF LIQUID PRESSURE WITH DENSITY.
The greater the density of the liquid, the greater the pressure and vice versa.
- PRESSURE AT ANY POINT OF THE LIQUID.
The pressure at any point in a liquid acts equally in all directions.
- A LIQUID FINDS ITS OWN LEVEL.
For any liquid the pressure at any point within its varies only with the vertical depth of the point below the surface of the liquid.
The liquid stands at the same vertical height in all the tubes whatever their shapes.
CALCULATION OF PRESSURE IN LIQUIDS.
Mass x acc.due to gravity = weight.
Mg= w
Weight = S gh
P = F/A = SAhg/A
P = S gh
Where S = Density of liquids.
g = acc.due to gravity
h = height/depth.
Example:
Given that the density of the liquid is 1000g/m contained to a height of 4m, calculate the pressure exerted by the liquid column is acceleration due to gravity, g = 10m/s2
Data:
S= 1000k/m2
H= 4m
G= 10m/s2
P = Sgh
= 1000 x 10 x 4
= 40,000 n/m2
MEASUREMENTS.
Measure heights h1 and h2 with reference to the surface of separation at B such that B and C are on the same horizontal level of the same liquid, this is true that the pressures at B and C are equal, since the atmospheric pressure is same on each side of the U-tube.
CONCLUSION:
The pressure at B due to liquid column AB equals to the pressure at C due to liquid column CD.
Let S1 be density of water and S2 be density of paraffin.
S1 h1g = S2 H2g
S1 H1 = S2 H2
S1 = S2 H2/ H2
SI/S2 = H2/H1
Note: If the density of water is 1000kg/m2, h1 and h2 are measured, then s2 can be calculated. An inverted U-tube known as Hare’s apparatus is used to compare the densities of two liquids which mix together.
Dip the two open tubes of Hare’s apparatus into beakers containing water and the liquid of the unknown density when the clip is opened to suck out the air, the liquids use to different heights and then remain there when the clip is closed.
– Measure h1 and h2.
Then P = S1h2g
S1h1g = S2h2g
S1h1 = S2h2
S1/h2 = h2/h1
Unknown density S2 can be calculated.
TRANSMISSION OF PRESSURE IN FLUIDS.
Liquids and gases are fluids because they can flow. Pascal’s principle states that ‘’When pressure is applied at any point on the surface of a fluid contained in closed vessel, the pressure is transmitted undiminished to all parts of the fluid and to the walls of the vessel.”
Uses of principle:
- Used in mechanical diggers
- Used in bull-dozers
- Used in hydraulic press
- Used in jacks
THE HYDRAULIC PRESS.
Pressure at small piston=F1/A1
Pressure at large piston=F2/A2
A force of 5N is applied to smaller piston. If the smaller piston has cross sectioned area of 0.001m2 and the larger piston has cross sectioned area of 0.1m2. Find the force of the larger piston.
f/a = F/A
5N/0.001 = F/0.1
5 X 0.1 = 0.001 F
F = 0.5/0.001 = 500N
F = 500N.
THE CAR BRAKES.
In modern cars, the braking systems retard the wheels equally to minimize the dangers of skidding or locking the wheels when the brakes are applied.
The driver presses the car using a small force to the master piston P which moves forward, the pressure exerted at the master piston P is transmitted, undiminished through the oil pipes to the piston of brake cylinder C, the piston N in the brake cylinder moves forward against the return spring thus bringing brake shoes B into contact with the brake drum D. This stops the wheels of the car.
MANOMETER.
A device used to measure gas pressure.
It consists of a U-tube containing a liquid, when both arms are open to the atmosphere, the same atmospheric pressure is exerted on the liquid surface A and B and there will be at the same horizontal level.
Side A is connected to the gas supply when the top is turned on, the gas exerts pressure on the surface A with the level B rises and A falls until the pressure at C in side the liquid becomes equal to the gas pressure i.e Shg, h is liquid heat.
Side A is connected to the gas supply, when the top is turned on the gas exerts pressure on surface A with the result that level B rises and A falls until the pressure at C inside the liquid becomes equal to the gas pressure i.e Shg, his liquid heat.
8.THE ATMOSPHERIC PRESSURE.
THE ATMOSPHERE.
Is a thick layer of air surrounding the earth’s surface.
The atmosphere contains the following gases:
- 80% of volume is Nitrogen.
- 20% of volume is Oxygen.
- Very small quantity is neon, krypton, carbon dioxide, xenon, argon, helium, hydrogen, ozone and water vapour.
Note – Air has weight. Due to its weight the atmosphere exerts a pressure on the earth’s surface is approximately 100,000 pa. This pressure acts not only on earth’s surface but also on the objects on the earth’s surface. The blood pressure is slightly greater than the atmospheric pressure.
EXPERIMENTS TO SHOW THAT THE ATMOSPHERE EXERTS PRESSURE.
- The crushing can experiment.
– Put some water in a can and boil for sometime while the can is open in order to drive off the air.
– Stop boiling and then close the can tightly with a stopper.
– Pour cold water over the can to cool it. The can collapses.
This is due to the fact that when boiling water is stopped, the can is full of steam, after inserting a stopper and cooling the can, the steam condenses to water of negligible volume compared to that of the steam. Since no air and steam is inside the can, the atmospheric pressure on the can causes its sides to collapse.
- The Magdeburg hemisphere experiment.
A scientist Magdeburg in 1651 performed an experiment to show that the atmosphere exerts pressure. The apparatus he used was called Magdeburg hemispheres.
The Magdeburg hemispheres were a pair of large copper hemisphere with mating rims, when the rims were sealed with grease and the air was pumped out the spheres contained a vacuum and could not be pulled apart by teams of horses.
- A glass tumbler experiment.
When the glass tumbler is filled with water to the brim and the card is placed over the top so that there is no air between the card and water, turning the glass tumbler upside down, the card firmly holds over the glass and the water does not fall out due to the fact that the upward air pressure on the card is greater than the downward pressure due to the short column of water in the tumbler.
BAROMETERS.
Is an instrument used to measure air pressure e.g. Atmospheric pressure.
- SIMPLE BAROMETER/MERCURY BAROMETER.
It uses mercury instead of water because mercury is denser than water.
Density of mercury = 13.6g/cm3
Density of water = 1g/cm3
Simple barometer has height of 76cm at sea level. Therefore, the atmospheric pressure at sea level is 76cm of mercury.
- FORTINS BAROMETER.
The commonly used barometer.
The glass tube T contains mercury M with vacuum above. A leather bag L at the base as a reservoir of mercury. A short fixed metal scale S graduated on one side in cm and on the other side in inches. A movable vernier scale V to read the height of the mercury level accurately.
A fixed ivory index I with a sharp point at the bottom corresponding to zero mark of the scale.
- ANEROID BAROMETER.
Mostly used in aeroplanes to record the air pressure at a certain attitude.
Note- A mountain climber at a certain height is under low pressure.
APPLICATIONS OF THE ATMOSPHERIC PRESSURE.
1.THE BICYCLE PUMP.
It consists of a hollow metal cylinder and a movable piston.
MODE OF ACTION.
When the opening of the pump is closed and the piston is withdrawn, a low pressure is created below the leather washer. The atmospheric pressure forces air into the pump through the space between the leather washer and the metal cylinder of the pump. When the piston is pushed forward the leather washer presses onto the sides of the metal cylinder of the pump and the tapped air below the leather washer is highly compressed and the bicycle tyre is filled with air through the valve.
- THE SYRINGE.
When the plunger is withdrawn, a low pressure region is created and water is forced into the syringe by atmospheric pressure. When the plunger is pushed down, water is pushed out. The syringe is commonly used for injecting medicine.
- THE COMMON OR LIFT PUMP.
MODE OF ACTION.
When a small pipe of the pump is dipped into the water and some water is poured on top of the piston to make it air tight.
- When the piston is pushed to the bottom of the barrel, valve A closes and air escapes through valve B.
- When the piston is raised, a low pressure is created between piston and valve A; Valve B closes due to atmospheric pressure on the piston. The atmospheric pressure force the water into the pump through valve A.
- When this action is performed continuously, the water above the piston is lifted up and pours out through a spout.
- THE FORCE PUMP.
This is a modification of common pump.
- THE SIPHON.
MODE OF ACTION.
- This is used to draw the liquid from one vessel into another.
- The pressure at E is greater than the atmospheric pressure.
- The excess pressure is the pressure due to the column DE of the liquid.
- This excess pressure forces the liquid out of the tube into another tube.
- THE AUTOMATIC FLUSHING TANK.
- This uses the siphon principle.
- Water fills the tank slowly and triggers the siphon action to start.
- The water flushes, the process repeats again and again.
- THE CHAIN AND BALL FLUSHING TANK.
MODE OF ACTION.
- It uses the siphon principle.
- Water fills the tank and the ball floats higher and higher and finally closes the valve top when the tank is full.
- The water is raised up when the chain C is pulled to fill bend N of siphon tube, until the tank is empty.
- The process repeats again.
THE ATMOSPHERE.
Is a thick layer of air surrounding the earth’s surface.
The atmosphere contains the following gases:
- 80% of volume is Nitrogen.
- 20% of volume is Oxygen.
- Very small quantity is neon, krypton, carbon dioxide, xenon, argon, helium, hydrogen, ozone and water vapour.
Note – Air has weight. Due to its weight the atmosphere exerts a pressure on the earth’s surface is approximately 100,000 pa. This pressure acts not only on earth’s surface but also on the objects on the earth’s surface. The blood pressure is slightly greater than the atmospheric pressure.
EXPERIMENTS TO SHOW THAT THE ATMOSPHERE EXERTS PRESSURE.
- The crushing can experiment.
– Put some water in a can and boil for sometime while the can is open in order to drive off the air.
– Stop boiling and then close the can tightly with a stopper.
– Pour cold water over the can to cool it. The can collapses.
This is due to the fact that when boiling water is stopped, the can is full of steam, after inserting a stopper and cooling the can, the steam condenses to water of negligible volume compared to that of the steam. Since no air and steam is inside the can, the atmospheric pressure on the can causes its sides to collapse.
- The Magdeburg hemisphere experiment.
A scientist Magdeburg in 1651 performed an experiment to show that the atmosphere exerts pressure. The apparatus he used was called Magdeburg hemispheres.
The Magdeburg hemispheres were a pair of large copper hemisphere with mating rims, when the rims were sealed with grease and the air was pumped out the spheres contained a vacuum and could not be pulled apart by teams of horses.
- A glass tumbler experiment.
When the glass tumbler is filled with water to the brim and the card is placed over the top so that there is no air between the card and water, turning the glass tumbler upside down, the card firmly holds over the glass and the water does not fall out due to the fact that the upward air pressure on the card is greater than the downward pressure due to the short column of water in the tumbler.
BAROMETERS.
Is an instrument used to measure air pressure e.g. Atmospheric pressure.
- SIMPLE BAROMETER/MERCURY BAROMETER.
It uses mercury instead of water because mercury is denser than water.
Density of mercury = 13.6g/cm3
Density of water = 1g/cm3
Simple barometer has height of 76cm at sea level. Therefore, the atmospheric pressure at sea level is 76cm of mercury.
- FORTINS BAROMETER.
The commonly used barometer.
The glass tube T contains mercury M with vacuum above. A leather bag L at the base as a reservoir of mercury. A short fixed metal scale S graduated on one side in cm and on the other side in inches. A movable vernier scale V to read the height of the mercury level accurately.
A fixed ivory index I with a sharp point at the bottom corresponding to zero mark of the scale.
- ANEROID BAROMETER.
Mostly used in aeroplanes to record the air pressure at a certain attitude.
Note- A mountain climber at a certain height is under low pressure.
APPLICATIONS OF THE ATMOSPHERIC PRESSURE.
1.THE BICYCLE PUMP.
It consists of a hollow metal cylinder and a movable piston.
MODE OF ACTION.
When the opening of the pump is closed and the piston is withdrawn, a low pressure is created below the leather washer. The atmospheric pressure forces air into the pump through the space between the leather washer and the metal cylinder of the pump. When the piston is pushed forward the leather washer presses onto the sides of the metal cylinder of the pump and the tapped air below the leather washer is highly compressed and the bicycle tyre is filled with air through the valve.
- THE SYRINGE.
When the plunger is withdrawn, a low pressure region is created and water is forced into the syringe by atmospheric pressure. When the plunger is pushed down, water is pushed out. The syringe is commonly used for injecting medicine.
- THE COMMON OR LIFT PUMP.
MODE OF ACTION.
When a small pipe of the pump is dipped into the water and some water is poured on top of the piston to make it air tight.
- When the piston is pushed to the bottom of the barrel, valve A closes and air escapes through valve B.
- When the piston is raised, a low pressure is created between piston and valve A; Valve B closes due to atmospheric pressure on the piston. The atmospheric pressure force the water into the pump through valve A.
- When this action is performed continuously, the water above the piston is lifted up and pours out through a spout.
- THE FORCE PUMP.
This is a modification of common pump.
- THE SIPHON.
MODE OF ACTION.
- This is used to draw the liquid from one vessel into another.
- The pressure at E is greater than the atmospheric pressure.
- The excess pressure is the pressure due to the column DE of the liquid.
- This excess pressure forces the liquid out of the tube into another tube.
- THE AUTOMATIC FLUSHING TANK.
- This uses the siphon principle.
- Water fills the tank slowly and triggers the siphon action to start.
- The water flushes, the process repeats again and again.
- THE CHAIN AND BALL FLUSHING TANK.
MODE OF ACTION.
- It uses the siphon principle.
- Water fills the tank and the ball floats higher and higher and finally closes the valve top when the tank is full.
- The water is raised up when the chain C is pulled to fill bend N of siphon tube, until the tank is empty.
- The process repeats again.
9.TEMPERATURE AND THERMOMETER.
Temperature is the degree of hotness or coldness of the body is called temperature.
SI unit of temperature are degrees centigrade.
Other units are Kelvin (K) and Fahrenheit (◦F)
The degree centigrade has lowest fixed point called ice point at 0◦ and the upper fixed point is the boiling point of water at 100◦C
In the Fahrenheit scale the lowest fixed point is 32◦F and the upper fixed scale is 212◦F
Note- There are 100 equal divisions in celcius scale but in Fahrenheit scale there are 180 equal divisions. These are called fundamental intervals.
CONVERSION OF SCALES.
Since celcius and Fahrenheit fundamental intervals are divided into 100 and 180 equal divisions respectively.
Consider two thermometers which are dipped in a hot liquid and their readings are X◦c and Y◦f.
The relation between Xºc and YºF is as follows:
x-0/100 = y-32/180
x = 100(y-320)/180
=5/9 (y-32)
C = 5/9 (F-32)
9/5c = F-32
F= 9/5c = F-32
F = 9/5(C + 32)
Question:
Change the following to ºc:
- 98º F
= 5/9(98- 32)
= 5/9 x 66
= 110/ 3
= 36.7ºC
Change the following to ºF
- 20ºC
= 9/5c + 32
= 9/5 x 20 + 32
= 36 + 32
= 68 ºF
THE THERMOMETER:
Is an instrument used to measure the temperature of the body. They are made by putting thermometric liquid inside the glass tube (alcohol) or mercury.
TYPES OF THERMOMETER:
-
CLINICAL THERMOMETER:
- The thermometer is used for measuring the temperature of the human being.
- The normal human being temperature is 36.9ºC. For this reason the clinical thermometer is graduated from 35 – 43ºC
- MERCURY IN GLASS THERMOMETER:
- The thermometer which contains mercury is called mercury in glass thermometer.
ADVANTAGES OF MERCURY AS A THERMOMETRIC LIQUID:
- Mercury boils at 360ºC (high boiling point.)
- It doesn’t wet the glass tube.
- It is opaque.
- It is a good conductor of heat and temperature.
- It expands steadily.
DISADVANTAGES OF MERCURY:
- It freezes at -39ºc lower freezing point.
- Has very small freezing capacity.
ADVANTAGES OF ALCOHOL:
- It freezes at -112ºc.
- It expands rapidly.
DISADVANTAGES OF ALCOHOL:
- Has low boiling point of 78ºc.
- It wets the glass tube.
- It is colorless.
- It is a bad conductor of heat and temperature.
DISADVANTAGE OF WATER:
- It wets the glass tube.
- It boils and freezes easily.
- Abnormal expansion.
- Is colorless.
- Bad conductor of heat and temperature.
- Has large heat capacity.
CONVERSION OF TEMPERATURE FROM UNDERGRADUATED THERMOMETERS.
Consider two thermometers: Celcius and Fahrenheit
In celcius scale we have 100 equal intervals and in Fahrenheit scale there are 180 equal intervals.
Temperature in ºC
x/y x 100ºC
Temperature in ºF
x/y x 180ºF
Example 1:
In an undergraduated thermometer, the length between the lower fixed point and the upper fixed is 65cm. The undergraduated thermometer is immersed in a hot liquid and the length of a thermometric liquid is 32cm. Find the temperature in ºc and ºf.
x/y x 100ºc
32/65 x 100 = 640/13 = 49.2ºc
x/y x 180ºf
32/65 x 180 = 1152/13 = 88.6ºf
Temperature is the degree of hotness or coldness of the body is called temperature.
SI unit of temperature are degrees centigrade.
Other units are Kelvin (K) and Fahrenheit (◦F)
The degree centigrade has lowest fixed point called ice point at 0◦ and the upper fixed point is the boiling point of water at 100◦C
In the Fahrenheit scale the lowest fixed point is 32◦F and the upper fixed scale is 212◦F
Note- There are 100 equal divisions in celcius scale but in Fahrenheit scale there are 180 equal divisions. These are called fundamental intervals.
CONVERSION OF SCALES.
Since celcius and Fahrenheit fundamental intervals are divided into 100 and 180 equal divisions respectively.
Consider two thermometers which are dipped in a hot liquid and their readings are X◦c and Y◦f.
The relation between Xºc and YºF is as follows:
x-0/100 = y-32/180
x = 100(y-320)/180
=5/9 (y-32)
| C = 5/9 (F-32) |
9/5c = F-32
F= 9/5c = F-32
| F = 9/5(C + 32) |
Question:
Change the following to ºc:
- 98º F
= 5/9(98- 32)
= 5/9 x 66
= 110/ 3
= 36.7ºC
Change the following to ºF
- 20ºC
= 9/5c + 32
= 9/5 x 20 + 32
= 36 + 32
= 68 ºF
THE THERMOMETER:
Is an instrument used to measure the temperature of the body. They are made by putting thermometric liquid inside the glass tube (alcohol) or mercury.
TYPES OF THERMOMETER:
- CLINICAL THERMOMETER:
- The thermometer is used for measuring the temperature of the human being.
- The normal human being temperature is 36.9ºC. For this reason the clinical thermometer is graduated from 35 – 43ºC
- MERCURY IN GLASS THERMOMETER:
- The thermometer which contains mercury is called mercury in glass thermometer.
ADVANTAGES OF MERCURY AS A THERMOMETRIC LIQUID:
- Mercury boils at 360ºC (high boiling point.)
- It doesn’t wet the glass tube.
- It is opaque.
- It is a good conductor of heat and temperature.
- It expands steadily.
DISADVANTAGES OF MERCURY:
- It freezes at -39ºc lower freezing point.
- Has very small freezing capacity.
ADVANTAGES OF ALCOHOL:
- It freezes at -112ºc.
- It expands rapidly.
DISADVANTAGES OF ALCOHOL:
- Has low boiling point of 78ºc.
- It wets the glass tube.
- It is colorless.
- It is a bad conductor of heat and temperature.
DISADVANTAGE OF WATER:
- It wets the glass tube.
- It boils and freezes easily.
- Abnormal expansion.
- Is colorless.
- Bad conductor of heat and temperature.
- Has large heat capacity.
CONVERSION OF TEMPERATURE FROM UNDERGRADUATED THERMOMETERS.
Consider two thermometers: Celcius and Fahrenheit
In celcius scale we have 100 equal intervals and in Fahrenheit scale there are 180 equal intervals.
Temperature in ºC
x/y x 100ºC
Temperature in ºF
x/y x 180ºF
Example 1:
In an undergraduated thermometer, the length between the lower fixed point and the upper fixed is 65cm. The undergraduated thermometer is immersed in a hot liquid and the length of a thermometric liquid is 32cm. Find the temperature in ºc and ºf.
x/y x 100ºc
32/65 x 100 = 640/13 = 49.2ºc
x/y x 180ºf
32/65 x 180 = 1152/13 = 88.6ºf
Thank for the topics
ReplyDelete